X chromosome inactivation is a critical biological process that ensures females, who possess two X chromosomes, can regulate gene expression effectively. This mechanism, discovered through years of research, holds significant implications for understanding chromosomal disorders such as Fragile X Syndrome and Rett syndrome. Notably, Jeannie Lee of Harvard Medical School has made substantial contributions to this field, exploring how the inactivation happens at the cellular level. By identifying the roles of various molecules, including Xist, researchers are unlocking new pathways for genetic therapies aimed at alleviating the symptoms of these conditions. As the scientific community delves deeper into X chromosome inactivation, the potential to develop innovative treatments continues to expand, offering hope for many affected by genetic disorders.
The phenomenon of X chromosome inactivation, often referred to as lyonization, is essential for maintaining genetic balance in female cells. This process compensates for the difference in X chromosome quantity between males and females, rendering one of the X chromosomes silent and effectively balancing gene dosage. Insights gained from research in this area shed light on significant issues linked to chromosomal abnormalities and intellectual disabilities. In particular, studies led by experts at prestigious institutions like Harvard are paving the way for cutting-edge interventions, including Fragile X treatment and advancements in Rett syndrome research. As scientists unveil the complexities behind gene silencing and activation, they are working towards groundbreaking genetic therapies with the potential to transform lives.
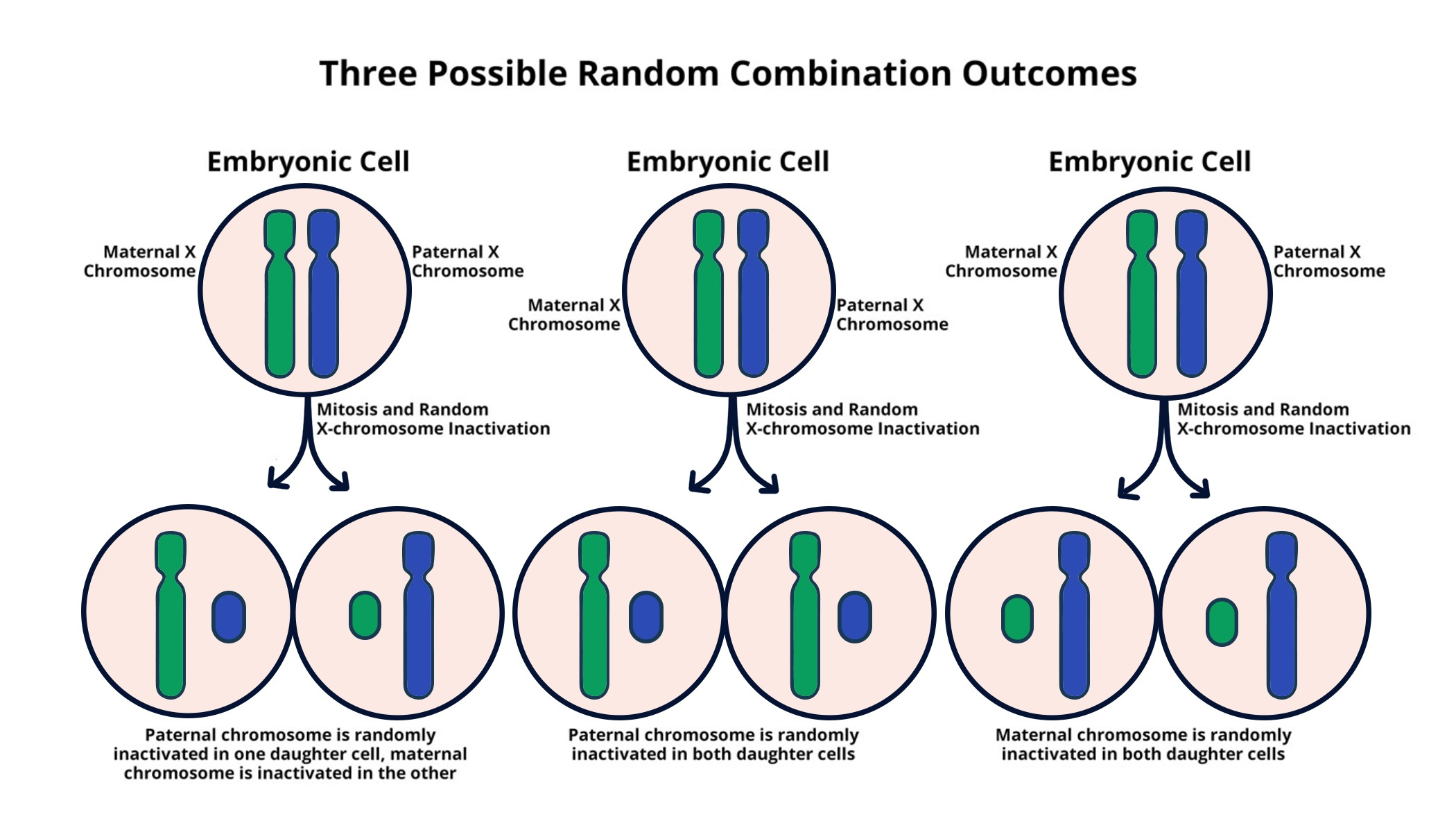
Understanding X Chromosome Inactivation Mechanisms
X chromosome inactivation (XCI) represents a pivotal biological mechanism that enables female mammals to balance gene expression between males and females. Since females possess two X chromosomes and males have one, nature devised a strategy to prevent females from over-expressing X-linked genes. The process is initiated by the gene Xist, which produces a long non-coding RNA essential for silencing one of the X chromosomes in each cell. This silencing is crucial to ensure that both sexes express similar levels of gene products, effectively preventing the phenotypic consequences of having an additional X chromosome.
Recent studies, particularly those from Jeannie Lee’s lab at Harvard Medical School, shed light on the intricacies of how this inactivation unfolds. Lee and her team discovered that the inactivation is facilitated by a gelatinous substance encapsulating the chromosomes, which functions to segregate them and maintain order within the cellular environment. This finding not only enhances our understanding of chromosomal architecture but also opens potential therapeutic avenues by targeting the processes involved in XCI.
The Role of Jeannie Lee’s Research in Genetic Therapies
Jeannie Lee’s relentless quest to unveil the mysteries of X chromosome inactivation has positioned her at the forefront of genetic therapy innovations. After years of funding and research, her team has made significant strides towards translating their findings into practical treatments for X-linked disorders such as Fragile X syndrome. They are exploring ways to reactivate the silenced X chromosome to express healthy copies of genes that have been adversely affected by mutations. This could mean a revolution in how conditions like Fragile X and Rett syndrome are treated, converting what was once thought to be permanent damage into a treatable condition.
The methodologies developed in Lee’s lab aim to effectively ‘unsilence’ genes located on inactive X chromosomes, showcasing the potential to restore proper gene function in patients with genetic disorders. This research holds promise not only for females but also for males who carry mutations on their single X chromosome. By unlocking the therapeutic potential of XCI, Lee’s work could pave the way for groundbreaking genetic therapies that address a wide range of chromosomal disorders.
The Intersection of Chromosomal Disorders and Therapeutic Strategies
Chromosomal disorders, particularly those associated with the X chromosome, have long posed challenges for genetic research and treatment. Conditions such as Fragile X syndrome exhibit significant variability in symptoms, influenced heavily by the genetic and environmental interplay. With advances in understanding X chromosome inactivation, researchers are hopeful that targeted strategies can be developed to treat these conditions more effectively. The key lies in leveraging knowledge about silenced genes and how they can be activated to regain functionality.
Lee’s exploration into the molecular dynamics of XCI has implications beyond just basic biology; it hints at viable therapeutic strategies that could alleviate the burden of various X-linked disorders. By focusing on restoring function to genes that have been silenced due to mutations, researchers are moving towards the development of new treatments that could transform the lives of countless individuals affected by these chromosomal disorders.
Innovative Approaches in Rett Syndrome Research
Rett syndrome, a neurodevelopmental disorder predominantly affecting females, has garnered significant attention within the field of genetic research due to its X-linked nature. The exploration of X chromosome inactivation provides valuable insights into potential therapies, particularly those aimed at mitigating the effects of mutations within the MECP2 gene, which is crucial for normal brain development. Jeannie Lee’s research indicates that understanding the mechanisms behind XCI can lead to the discovery of novel intervention strategies and, ultimately, treatments.
By unlocking insights into how genes can be reactivated after being inactivated, researchers aim to develop therapeutic compounds that can target individuals suffering from Rett syndrome. This promising area of study emphasizes the importance of genetic therapies that can address the underlying causes of neurodevelopmental disorders, offering hope for better disease management and improved outcomes for patients.
Advancements in Fragile X Treatment Through Genetic Research
Fragile X syndrome, characterized by intellectual disabilities and behavioral issues, is the most common inherited cause of these disorders. Jeannie Lee’s lab is pioneering research that focuses on unsilencing the genes on the inactivated X chromosome, which may hold the key to effective treatments for those affected. By employing innovative methods to target the complex mechanisms of XCI, researchers are hopeful that they can reactivate the healthy gene copies that are crucial for normal development and cognition.
The potential breakthroughs from Lee’s research not only aim to treat Fragile X syndrome but also pave the way for new paradigms in genetic therapies that could expand to other conditions. As scientists refine their techniques, the goal of delivering effective treatments to patients with fragile X and degeneration disorders becomes less distant, reflecting a new era in the intersection of genetic research and clinical application.
Exploring the Gelatinous Substance in Chromosomal Biology
The unique Jell-O-like substance that surrounds chromosomes plays a critical role in cellular organization and gene accessibility. This viscous medium not only separates chromosomes but also facilitates the process of X chromosome inactivation by allowing Xist RNA to modify its properties. Understanding how this gelatinous packaging interacts with chromosomal structures provides insights into how cells manage genetic material effectively and responds to mutations.
As researchers investigate the properties of this gelatinous substance further, the potential implications for enhancing gene therapy become apparent. By modifying the stiffness or fluidity of this medium surrounding chromosomes, scientists might create opportunities to improve access to silenced genes, thereby enabling better methodologies for treating genetic disorders caused by X-linked mutations.
Clinical Implications of X Chromosome Reactivation
The clinical implications of reactivating the inactivated X chromosome are profound, particularly for individuals suffering from X-linked disorders such as Fragile X and Rett syndrome. As scientists like Jeannie Lee make progress in this area, the possibility of converting established genetic theories into real-world applications becomes a tangible goal. The aim is to develop viable treatment options that can restore normal gene activity without adverse effects on healthy genes.
The challenge moving forward involves the careful design and testing of therapies aimed at reactivating silenced genes. This requires a multidisciplinary approach that incorporates genetics, molecular biology, and clinical insights to ensure safety and efficacy. The potential benefits are significant: therapies that restore function could not only enhance the lives of patients but also reshape our understanding of genetic disorders and their treatment.
Developing Safety Studies for Genetic Therapies
As hope builds around the reactivation of silenced genes tied to X-linked disorders, it is essential to conduct extensive safety studies to assess the viability of these therapies. Researchers in Lee’s lab are committed to refining their approaches and conducting rigorous trials that evaluate the effects of proposed treatments on both individuals with genetic conditions and unaffected genes. Ensuring that new therapies are not only effective but also minimize risks is crucial for successful clinical application.
The outcome of these safety studies will play a pivotal role in determining which genetic therapies can progress to clinical trials. With ongoing advancements in understanding X chromosome inactivation and gene reactivation, researchers envision a future where targeted treatments could significantly diminish the impact of chromosomal disorders like Fragile X and Rett syndrome, ultimately changing the narrative for countless patients and families.
Future Directions in Genetic and Cellular Research
The future of genetic and cellular research is poised for exciting developments, particularly in the realm of understanding and manipulating chromosome function. With the work of researchers like Jeannie Lee leading the way, promising strategies for gene therapy and the treatment of chromosomal disorders will likely continue to emerge. Focusing on the intricate mechanisms underlying X chromosome inactivation could provide breakthroughs not only for X-linked conditions but also for a broader scope of genetic diseases.
As progress continues in functional genomics and genetic therapies, the convergence of scientific knowledge will play a crucial role in formulating innovative solutions to longstanding medical challenges. The intersection of chromosomal biology with therapeutic strategies signifies a promising direction for future research, potentially revolutionizing our approach to genetic disorders and ushering in a new era of personalized medicine.
Frequently Asked Questions
What is X chromosome inactivation and why is it important?
X chromosome inactivation is a biological process that occurs in females, where one of the two X chromosomes is randomly silenced to prevent the production of excessive gene products. This process is crucial for ensuring that the dosage of X-linked genes is balanced between males, who have one X chromosome, and females, who have two. Understanding this mechanism is vital for addressing chromosomal disorders associated with the X chromosome.
How does X chromosome inactivation relate to Fragile X syndrome treatment?
X chromosome inactivation is particularly significant in the context of Fragile X syndrome, a genetic disorder caused by mutations on the X chromosome. Recent research, including studies by Jeannie Lee at Harvard Medical School, suggests that methods to unsilence inactivated X chromosomes could restore function to the healthy gene in individuals affected by Fragile X syndrome, potentially leading to new treatments.
What role does Jeannie Lee play in research on X chromosome inactivation?
Jeannie T. Lee is a leading researcher in the field of genetics at Harvard Medical School, focusing on X chromosome inactivation. Her lab is exploring the mechanisms behind this process and its implications for therapeutic applications, specifically for conditions like Fragile X syndrome and Rett syndrome. Lee’s work could pave the way for innovative genetic therapies targeting these chromosomal disorders.
Can X chromosome inactivation strategies assist in Rett syndrome research?
Yes, strategies developed to manipulate X chromosome inactivation are being investigated in Rett syndrome research. Given that Rett syndrome is often linked to mutations on the X chromosome, unlocking the inactivated X chromosome could allow access to the healthy gene copy, providing potential avenues for treatment and reversal of symptoms in affected patients.
How does the jelly-like substance contribute to X chromosome inactivation?
The jelly-like substance surrounding chromosomes, described by Jeannie Lee, plays a vital role in X chromosome inactivation. It creates a flexible environment that allows regulatory molecules, including Xist RNA, to penetrate and modify its properties. This alteration is essential for effectively silencing one of the X chromosomes in females, preventing genetic disorders caused by excess gene expression.
What is the significance of the ‘Aha’ moment in X chromosome inactivation research?
The ‘Aha’ moment in Jeannie Lee’s research signifies a breakthrough in understanding X chromosome inactivation, revealing how the process can be manipulated for therapeutic use. This realization opens up new possibilities for treating genetic disorders linked to the X chromosome, such as Fragile X syndrome and Rett syndrome, highlighting a transition from basic research to potential clinical applications.
Are there potential side effects of therapies targeting X chromosome inactivation?
Preliminary studies on therapies that aim to unsilence inactivated X chromosomes indicate that they may restore function to mutated genes without significantly impacting healthy genes on the X chromosome. This suggests a promising approach with minimal side effects, but ongoing research is necessary to fully understand the implications and ensure safety in clinical settings.
What challenges remain in understanding X chromosome inactivation?
Despite recent advancements, several challenges remain in fully deciphering X chromosome inactivation. Questions still exist about why liberating inactivated X chromosomes primarily aids in restoring mutated gene function while sparing healthy genes. Further investigation is needed to refine therapeutic approaches and clarify the underlying mechanisms governing gene utilization in this context.
| Key Points | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes that must inactivate one to avoid excess gene expression. |
| Role of Xist Gene | The Xist gene produces RNA that interacts with the surrounding chromosomal substance to initiate inactivation. |
| Mechanism of Inactivation | Xist alters the properties of the Jell-O-like substance surrounding the X chromosome, facilitating gene silencing. |
| Potential Therapies | Research may lead to treatments for Fragile X Syndrome and Rett Syndrome by ‘unsilencing’ inactivated X-linked genes. |
| Clinical Applications | Ongoing studies are focused on safely optimizing methods to move towards clinical trials for these conditions. |
| Future Directions | Understanding X chromosome inactivation could free atypical X chromosomes and treat genetic disorders with fewer side effects. |
Summary
X chromosome inactivation is a crucial biological process that allows females to manage the presence of two X chromosomes by silencing one to avoid gene dosage issues. Recent research led by Jeannie T. Lee has unveiled the intricacies of this process, providing the groundwork for potential therapies aimed at treating genetic disorders linked to mutations on the X chromosome. By utilizing the Xist gene’s ability to manipulate the surrounding chromosomal environment, scientists are paving the way for innovative treatments for conditions like Fragile X and Rett syndrome. With ongoing studies focused on clinical applications, the future holds promising possibilities for those affected by X-linked genetic disorders.